WRAP Certification 窶?Passport for Textile and Garment Enterprises in the Time of Integration In the article below we will provide some information about the world’s largest independent certification program focusing on the field of apparel, footwear and sewing products (WRAP). 1. What is WRAP? WRAP stands for Worldwide Responsible Accredited Production – the name...
]]>WRAP Certification 窶?Passport for Textile and Garment Enterprises in the Time of Integration
1. What is WRAP?

Security
2. Benefits of Applying for WRAP Certification and Being Certified:
3. Three levels of WRAP certification:
4. Kim Sora was honored to receive WRAP Certification

Why do you need to use standard medical masks and how to choose the right one? In the article below, we will provide information about a type of European quality standard and introduce to you a type of medical mask that meets the recommended standard for use, which is a type of medical mask that...
]]>Why do you need to use standard medical masks and how to choose the right one?
Learn about EN 14683 standard
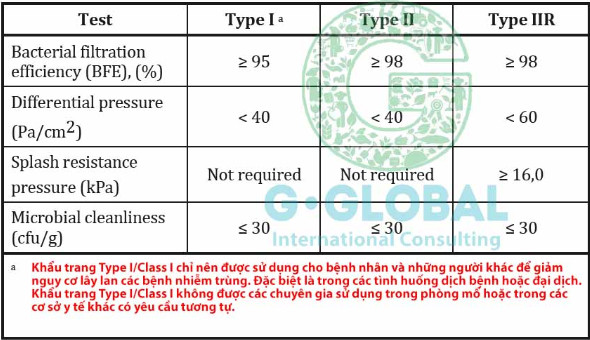
How to choose a standard mask


In addition, Kim Sora medical masks are certified:
Latest update of Kim Sora’s ISO 13485:2016 certificate Kim Sora is honored to continue to be evaluated and in accordance with the requirements of international standard ISO 13485:2016 for the field of production and distribution of medical masks issued by the Quality Testing and Certification Center (TQC). ). In the article below, we would like...
]]>Latest update of Kim Sora’s ISO 13485:2016 certificate
Kim Sora is honored to continue to be evaluated and in accordance with the requirements of international standard ISO 13485:2016 for the field of production and distribution of medical masks issued by the Quality Testing and Certification Center (TQC). ). In the article below, we would like to provide some information related to the international certificate ISO 13485: 2016.
What is ISO 13485:2016 standard?
The latest version is ISO 13485:2016, a set of international standards for management systems applied in the field of manufacturing and trading of tools; medical supplies. Applied to ensure the ability to provide products that meet customer requirements and legal regulations.

How important is ISO 13485:2016?
The company’s medical equipment products benefit greatly from the input stage to the production process, including a quality management system certified to ISO 13485 standards. Below are a few factors that show its importance. The importance and benefits of ISO 13485 when businesses apply:
Provide safe products for users
Create competitive advantage, enhance brand, can be easily exported
Save costs, increase revenue and profits, minimize risks
Activities are managed systematically, helping to control product quality and safety
Improve production efficiency and product quality
Control hazards, control hygiene and contamination and have a specific implementation plan
Labor productivity increases
Improve the ability to meet customer requirements. Meet national and international regulations for medical products
Improve competitiveness for businesses and expand markets
Improve the efficiency of the current management system. Convenient integration with other management systems (ISO 9001, ISO/IEC 17025, ISO 14001).
ISO 13485:2016 窶?Latest version
Standard on Safety Management System for Medical Products is part of the ISO 13485:2003 standard set, the first version of which was issued by the international standardization organization ISO in July 2003. (Equivalent to the International Standard (TCVN ISO 13485:2004) ISO 13485:2012 (or BS EN ISO 13485:2012).
ISO 13485:2016 窶?The latest version was released to replace the old version ISO 13485:2003. This standard was published on 1 March 2016. There is a 3-year transition period from the old version to the new version for manufacturers and other organizations. That is until February 28, 2019 when ISO 13485:2003 expires. It also means that updating to the new version from the old version ISO 13485:2003 and the related European standard: EN ISO 13485:2012 needs to be done no later than this date.
Kim Sora is assessed with international certificate ISO 13485:2016
Continuing the quality standards evaluated in previous years, Kim Sora Co., Ltd. is honored to continue to be evaluated and in accordance with the requirements of international standard ISO 13485:2016 effective from September 25. March 2023 to March 24, 2026. This demonstrates the development and continuous improvement of product quality and production processes to bring quality – safe – effective products to Kim Sora’s customers.
In addition, Kim Sora medical masks are certified:

ASTM F2100-19 Level 1,2,3: is a standard of the American Institute of Standards and Technology (ASTM International) for medical masks used in medical procedures and infection prevention.
ISO 9001:2015: is a standard system for product quality management;
FDA: is to monitor and evaluate food quality; Whether the pharmaceutical product meets the criteria for import into the US?
CE: is a document indicating that the product has met health protection standards; safety and environmental protection in accordance with European Unionツ (EU) legislation and will be marketed within the European Economic Areaツ (EEA).
Please contact Kim Sora immediately for advice and to order today.
Email: [email protected]
Hotline: 0905 986 797
Fanpage: Kim Sora Mask
]]>